Human iPSCs in inherited cardiomyopathies
Therefore, isogenic controls generated by CRISPR-Cas9 technology are used for direct comparison of iPSC derived CMs. Other concerns such as the acceleration of maturation and the formation of sarcomeres in vitro are addressed by mechanical and electrical stimulation protocols. Our goals are focused on the characterization of pathological changes displaying clinical features of hiPSC derived CM from different cardiomyopathy patients compared to their isogenic controls.
Question: What are human induced pluripotent stem cells (hiPSCs)?
Answer: Human iPSCs are embryonic stem-like cells that have been reprogrammed from somatic cells (e.g. dermal fibroblasts) using a transfection vector and a well-defined set of embryonically expressed pluripotency factors (four `Yamanaka Factors`: OCT-4, SOX-2, Klf4, c-Myc). Similar to human embryonic stem cells (ESC), hiPSCs self-renew and can differentiate into cells of all three germ layers (endoderm, mesoderm, and ectoderm).
Question: What are inherited cardiomyopathies (CMPs)?
Answer: Inherited CMPs are diseases that affect the heart muscle leading to heart failure and sudden death. Different types of CMPs include hypertrophic CMP (HCM), dilated CMP (DCM) and arrhythmogenic (right ventricular) CMP (ACM) and even more rare subtypes.
Question: What is CRISPR/Cas9 technology?
Answer: CRISPR (clustered regularly interspaced short palindromic repeats)/Cas (CRISPR-associated) system has been developed as an efficient and versatile technology for genome editing in eukaryotic cells and whole organisms. This technology can be engineered to induce Cas9-mediated double-stranded breaks (DSBs) in a sequence-specific manner. The resulting DSB will either generate nonspecific mutations knocking out a gene through the error-prone NHEJ (nonhomologous end joining) pathway, or produce specific modifications dictated by an exogenous repair template through the HDR (homology-directed repair) pathway.
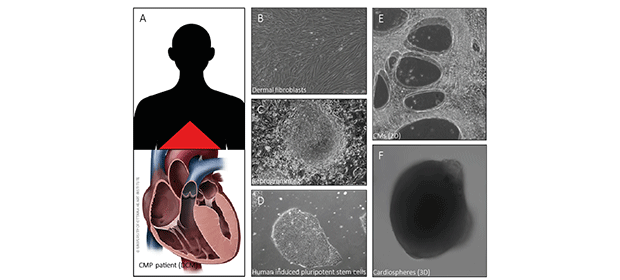
Figure: Inherited cardiomyopathy patients (e.g. dilated cardiomyopathy) donate skin samples to allow us the investigation of the disease specific mutation (A). Out of the skin we are obtaining dermal fibroblasts (B) to reprogram the cells (C) into patient specific human induced pluripotent stem cells (hiPSC, D). These hiPSCs are differentiated in vitro into cardiomyocytes (heart muscle cells, CMs) to generate a 2D monolayer of contracting CMs (E) or a 3D cardiosphere model (F).