This project aims to uncover how nerve growth factor (NGF)-induced signalling, particularly via interleukin-7 (IL-7), contributes to the development and resolution of chronic postsurgical pain (CPSP) following abdominal hernia repair. The goal is to identify mechanisms that can be targeted to improve pain outcomes after surgery.
Background
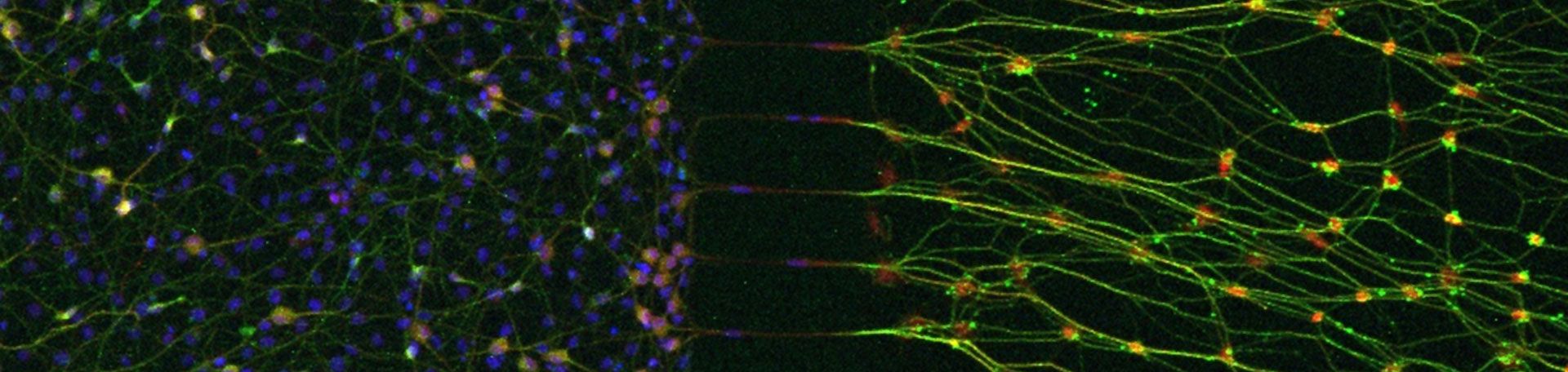
Chronic postsurgical pain (CPSP) is a frequent complication after abdominal surgeries, with over 50% of patients affected following hernia repair. NGF is a key mediator released early after tissue injury, sensitizing nociceptors and modulating pain pathways. While much is known about the effects of NGF on the cell bodies of sensory neurons, its actions at distal nerve terminals are poorly understood. Preliminary findings indicate that NGF stimulation induces IL-7 expression in axons, which attracts neutrophils to wound sites. Neutrophil accumulation is believed to play a dual role: it supports wound healing but may also promote persistent pain.
Research Objective
We aim to investigate how NGF-induced IL-7 signalling at peripheral nerve endings regulates neutrophil recruitment, neuroinflammation, and pain resolution after laparotomy.
Significance
By elucidating the role of NGF-IL-7 signalling in postsurgical pain, this project could pave the way for novel therapeutic strategies aimed at preventing CPSP. Limiting IL-7-mediated neutrophil recruitment may offer a targeted approach to enhance pain resolution and improve postoperative recovery for patients undergoing abdominal surgeries.
Research Team NP8
Head
PD Dr. rer. nat. Michael Briese
Institute of Clinical Neurobiology
University Hospital Würzburg
Prof. Dr. Nana-Maria Wagner, MD
Deputy Clinic Director of the Department of Anesthesiology, Intensive Care, Emergency and Pain Medicine
University Hospital Würzburg
Members of the team
Ankita Rawat, PhD Student


